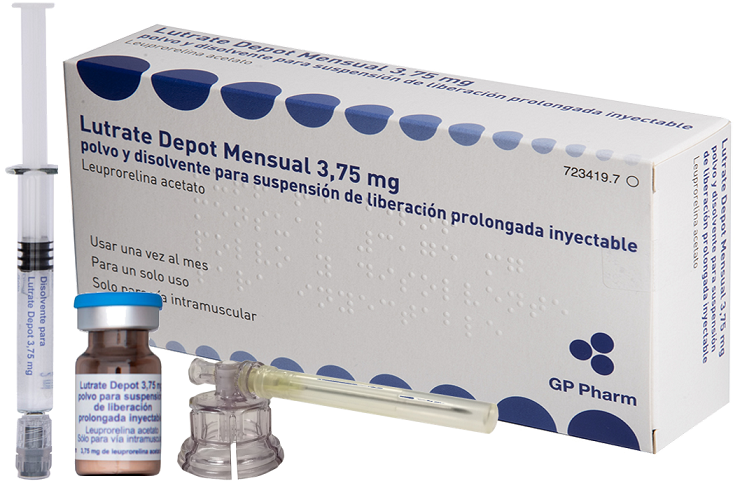
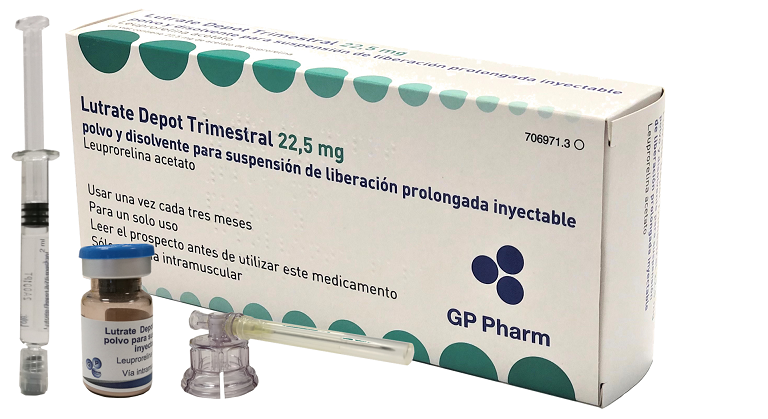
Ready to market products
Potential License Out Opportunities
Licensed-in products
For the Spanish Market
Research Projects
Opening Partnering for Co-Development & License
Icatibant is a synthetic decapeptide with a structure similar to bradykinin, but with 5 non-proteinogenic amino acids. It is used for symptomatic treatment of acute attacks of Hereditary AngioEdema (HAE)
LUTRATE® Depot Semestral (45,0 mg) es una nueva formulación de liberación prolongada de acetato de leuprorelina, un agonista de la LHRH, que se utiliza para el tratamiento de cáncer de próstata avanzado. El producto ha completado su desarrollo preclínico y está listo para entrar en las fases clínicas.