GP Pharm research team is highly specialized in the pharmaceutical encapsulation field using liposomes & microspheres. Our team has broad experience in the development and manufacturing of pharmaceutical injectable drugs including encapsulation of peptides, oligonucleotides and other APIs in liposomes and microspheres.

GP Pharm is also specialized in the development and manufacturing of freeze-dried pharmaceutical injectable products.
GP Pharm’s Research and Development areas work with the latest innovative technologies like UPLC, Quantitative HPTLC, HPLC, DSC, KF for water content determination, DLS and laser diffraction for particle size measurement, , Z potential, polymer analysis by GPC, viscosimetric analysis, UV, FT-IR, head space GC and other specific technologies for the analysis of liposomes and microspheres.
Due to these powerful research capacities, the company is able to assure fast and successful results in all steps of pharmaceutical product development and scale up processes from laboratory trials to clinical and commercial production.

As a result of R&D efforts and our proprietary technology platforms, GP Pharm is able to develop new generations of conventional pharmaceutical products by the incorporation of the corresponding APIs in microspheres or liposomes, improving the efficacy and the pharmaceutical behavior of existing conventional products in the market.
Microspheres
What are Microspheres and what are they used for?
A medicinal drug is normally administered in one dose at a given time, and then that dose has to be repeated several hours or days later to have a constant pharmacological effect. This administration pattern leads to poor control of drug delivery and important drug blood level oscillation. Especially for high potency products, this can often result in damaging side effects.
Consequently, increasing attention has been focused on drug delivery methods to administer high potent active ingredients for prolonged time periods in a time-controlled fashion. The main targets are: to reduce the undesired side effects, increase the time between drug administrations and achieve a deep control of drug delivery.
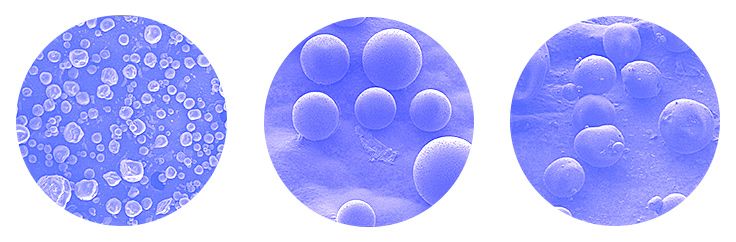
The primary method of accomplishing this controlled release is based on the incorporation of the active ingredients biodegradable polymeric microcapsules. In these microencapsulated products, the drug is entrapped in one polymeric matrix or coated by one polymeric shell. When the microencapsulation process yields small sized spherical shape particles, these particles are normally called microspheres.
Microsphere technological capabilities within GP Pharm
- GP-Pharm has experience obtaining Microspheres throughout different technologies such as: simple emulsion, double emulsion and co-acervation
- Expertise to manufacture hydro- and liposoluble products by use of different additives within the capsule surface
- Expertise in handling synthetic and natural biopolymers
- Ability to manufacture microspheres from 5 to 200 µm
- Ability to control release from 5-6 days up to 6 months
- Broad experience in scaling-up processes from lab to plant
- Wide experience in manufacturing at industrial level
- GP Pharm innovative technologies and products are patent covered.
Liposomes
What are Liposomes and what are they used for?
Liposomes are lipotropic and thermotropic liquid crystals composed by lipid bilayers of amphiphilic lipids that are thermodynamically stable. Liposomes are spontaneously formed by dispersing phospholipids in an aqueous medium and may be described as spherical, closed structures of colloidal dimensions and formed by one or more concentric bilayers that contain aqueous compartments. Liposomes have a great potential as drug delivery systems for parenteral delivery of drugs due to their unique properties that allow encapsulation of both lipid-soluble and water-soluble active ingredients.
Because of its structure and its design based on lipid components, liposomes are biocompatible, biodegradable and relatively non-toxic. The application of liposomes as carriers for drug delivery has been extensively studied. Liposomal products have been studied in addition to the administration of drugs, also in cosmetic formulations or for the diagnosis and various applications in the food industry.
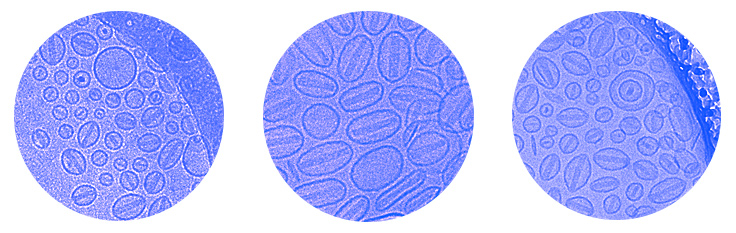
Liposome formulations have been widely investigated for delivery of anti-tumor substances, antimicrobial treatments for bacterial agents and diseases induced by viral parasites as well as for use as immunological adjuvants for vaccines. More recently numerous studies demonstrated the applicability of liposomes for gene therapy or for the use of antisense, small interfering RNA.
Currently, many clinical studies for liposome preparations are under research and various products have reached the market successfully.
Liposome technological capabilities within GP Pharm
- Development of in vitro release test in different buffers and human plasma.
- Liposomal Characterization by Freeze-fracture Electron Microscopy, Cryo-ElectronMicroscopy, TEM and SEM.
- Characterization of loaded drugs in liposomes by circular dichroism and X-Rays.
- DSC to determination of transition Temperatures.
- Determination of collapsing temperatures for freeze dried liposomal formulations.
- Development of freeze-dryer processes for liposomal formulations.
New Finished Form Formulations
GP Pharm expertise in formulation and manufacturing of difficult finished forms (lyophilized, solution, microspheres, liposomes) containing special Active Pharmaceutical Ingredients such as peptides, DNA, RNA, HPAPIs, cytotoxic, etc. opens and enormous potential of improved formulations more stable, more convenient to patients and with a more appropriate release delivery of the drug.