
Mission
To improve people's health and quality of life by manufacturing highly complex injectable products that are accessible to everyone.

Vision
To become the most reputable and reliable pharmaceutical company for our patients and partners, both nationally and internationally, providing the highest quality injectable products through sustainable growth that reduces environmental impact.

Culture
Our company culture is grounded in three essential pillars: Innovation, generating advancements to improve patients' lives and contribute to the progress of the pharmaceutical industry. Achievement, always driving excellence. The Team, strengthening talent and internal collaboration to develop a great team.
History of GP Pharm

Research, development and innovation
GP Pharm's research and development team is highly specialised in the microencapsulation of drugs using liposomes, lipid nanoparticles, and microspheres. Our team has extensive experience in the development and manufacture of injectable pharmaceutical products that include these technologies. The company is an expert in the microencapsulation of peptides, oligonucleotides, mRNAs, and other active ingredients.
Microspheres
What are microspheres and what are they used for?
Generally, pharmaceutical products must be administered several times a day over a determined period of time to achieve a constant pharmacological effect. This administration pattern can result in poor control of drug levels in the blood, leading to concentration fluctuations that may occasionally fall below the therapeutic efficacy values or above the toxicity levels of the drug in question. Especially in products with high pharmacological potency, this can lead to unwanted side effects for the patient.
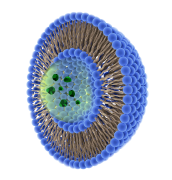
Liposomes and lipid nanoparticles
What are liposomes and what are they used for?
Liposomes are thermodynamically stable vesicular structures composed of lipid bilayers of amphiphilic lipids. Liposomes are formed spontaneously by the dispersion of phospholipids in an aqueous medium and can be described as spherical, closed, submicron-sized structures formed by one or more concentric lipid bilayers containing aqueous spaces between them. Liposomes have great potential as controlled drug delivery systems for parenteral administration thanks to their unique properties that allow the encapsulation of hydro- and fat-soluble substances. Therefore, it is an ideal release system for controlled releases with times of 1 to 4 days.
Technological platforms based on nano and microencapsulation
Nanotechnology/nanoencapsulation
With the technological advances of recent years, nanotechnology has shown enormous potential for clinical application, especially in the generation of lipid nanoparticles as vectors for drug delivery.
- nanoemulsions
- liposomes
- lipid nanoparticles

New types of microencapsulated formulations
GP Pharm's high experience in the development and production of final products (lyophilised, solutions, microspheres, and liposomes) containing active principles such as peptides, DNA, RNA, HPAPIs, cytotoxics, among others, opens up enormous potential for the new development of improved formulations with greater stability, more suitable for patients, and with a more controlled drug release.

Industrial facilities
GP Pharm's industrial facilities are located in an area of 35,000 square metres in Sant Quintí de Mediona (approximately 50 km west of Barcelona). Inaugurated in 2005, the project was designed considering a possible growth of the facilities in parallel to that of the company. In fact, in 2017 part of the facilities were expanded and renovated to double the manufacturing capacity and adapt to the growing demand. Currently, they occupy 3,651m² and the industry has 8 buildings, of which 3 are exclusively dedicated to the manufacture of pharmaceutical products. The rest is used for offices, laboratories, logistics, and supplies. Our R+D and quality control laboratories are located in the same industrial area for greater control in the development and validation of the analytical methods used in production control. The co-location of the R+D and production teams allows GP Pharm to establish an optimal collaboration to ensure a smooth transition from laboratory development to scale-up to industrial processes.
Areas of expertise
Medical department

The medical department designs, conducts, and coordinates the complete clinical development of our pharmaceutical products. The extensive know-how of our team in all areas of this process guarantees the correct development from the preclinical phase to phase IV studies, ensuring quality control.
They are also specialised in medical affairs, being a pillar for the product strategy and providing high value in scientific communication with all stakeholders, including national and international regulatory agencies.
Our team has extensive experience—academic profiles with master's degrees and doctorates—in the validation, design, and conduct of clinical studies, generation of scientific evidence, medical positioning of our products, communication, and scientific training. These functions mean that it works in constant collaboration with the different departments of the company, providing support, among others, to pharmacovigilance, market access, and regulatory aspects.
Our cornerstone is to ensure the health and well-being of patients from the initial phases of research to clinical practice.
Intellectual Property

GP Pharm develops innovative products and technologies in the field of the pharmaceutical industry. In order to protect the significant value of the company's intellectual property generated in its laboratories, the company has created a comprehensive international patent portfolio.
GP Pharm holds more than 100 patents and patent applications in more than 50 countries, protecting key products and technologies including, but not limited to, controlled drug release system formulations.
Supply chain

The supply chain of GP Pharm's pharmaceutical products is perfectly integrated: reception, identification, storage, order preparation, documentation, shipment, and distribution are perfectly monitored to have perfect traceability of the shipment of the product to the customer and ensure the quality and storage of it under the authorised conditions.
The company has extensive experience in shipping temperature-controlled products anywhere in the world, including ambient temperature (<25ºC), cold (between 2 and 8ºC), and/or below zero (-20ºC). We work with the best suppliers of active and passive temperature control systems, carefully choosing the most suitable one according to the shipping system to guarantee the quality of the final product at destination.
The company assumes responsibility for the preparation of export documentation according to the different regulations of each country to facilitate the import process for the customer and expedite shipping.
Customer Service

Our customer service, both nationally and internationally, fully monitors all sales processes, from the moment of receipt of the order to its delivery, to guarantee the best service and satisfaction to our customers. In this regard, we are committed to providing an exceptional level of service through our attention to detail and a deep understanding of the needs of each of our clients.
Regulatory Affairs

GP Pharm's regulatory affairs team has extensive experience in registration procedures worldwide, especially at the European level (NP, DCP, CP, MRP) and in the USA. It also has extensive experience in supporting registration procedures in other territories such as Canada, Australia, ASEAN, APAC, MENA, LATAM, among others.
GP Pharm's core capabilities include:
- Preparation of new dossiers in e-CTD format.
- Audits of registration dossiers and DMFs.
- Processing of authorisation processes and updating of registration dossiers in different regions.
- Support to clients in regulatory procedures in different territories.
Pharmacovigilance Department

The pharmacovigilance team works to ensure the safety and efficacy of our medicines during development, marketing, and the entire life cycle of the product, monitoring the adverse effects linked to our medicines and complying with the health obligations associated with pharmacovigilance.














